Correlation between AHSA1 expression and prognosis of hepatocellular carcinoma based on TCGA database analysis
-
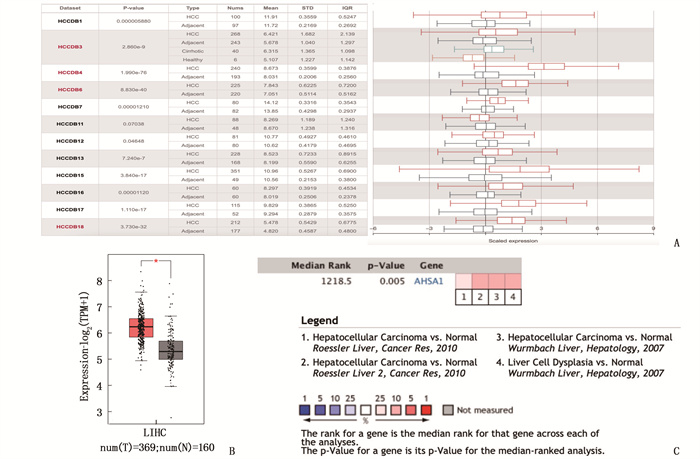
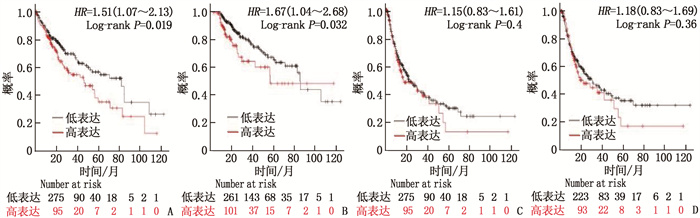
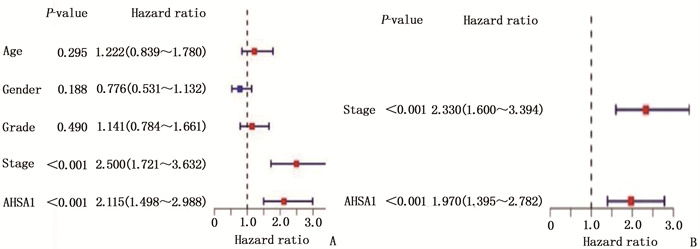
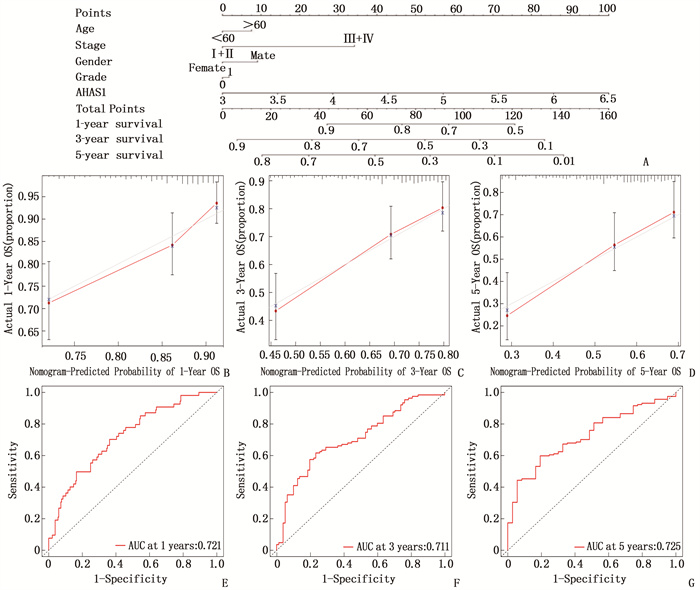
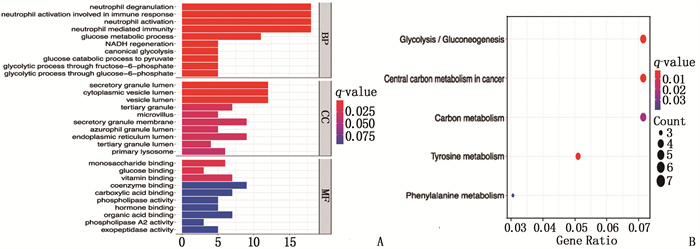
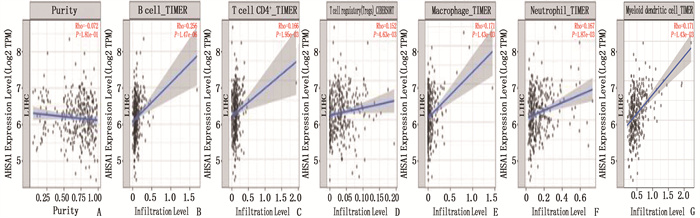
摘要:目的 探讨热休克90 kDa蛋白ATPase同源物1的激活因子(AHSA1)在肝细胞癌(HCC)中的表达,分析AHSA1表达水平与预后的关系。方法 利用HCCDB、GEPIA和Oncomine数据库分析AHSA1 mRNA在HCC组织及正常肝组织中的表达情况。从癌症基因组图谱(TCGA)下载HCC RNA-seq表达和临床信息,使用单变量、多变量Cox比例风险回归模型以及Kaplan-Meier图来评估AHSA1预测肝癌预后的价值。利用R软件构建基于AHSA1表达水平的列线图,绘制校准曲线评估实际生存和预测生存的一致性。通过基因本体论(GO)、京都基因和基因组百科全书(KEGG)基因集富集分析揭示与AHSA1相关的肿瘤相关生物学过程。通过TIMER2.0及GFPIA数据库分析HCC中AHSA1的表达与肿瘤免疫浸润的相关性。通过CMAP筛选出作用于AHSA1的小分子靶向药物。结果 HCC组织中AHSA1的表达水平高于正常组织,差异有统计学意义(P < 0.01)。AHSA1高表达与HCC预后较差有相关性(P < 0.05), AHSA1高表达是影响HCC患者总体生存期(OS)的独立因素(HR=1.970, P < 0.001)。列线图显示AHSA1基因表达与HCC风险存在相关性,其预测1、3、5年OS的曲线下面积(AUC)分别为0.721、0.711、0.725。校准图显示预测生存率曲线与实际生存率曲线具有较好的一致性。GO和KEGG富集分析显示, AHSA1可以通过介导中性粒细胞活化参与糖酵解、糖异生等生物学过程来促进肿瘤的进展。AHSA1 mRNA表达水平与B细胞、CD4+T细胞、调节性T细胞、巨噬细胞、中性粒细胞和树突状细胞的免疫浸润程度有相关性(P < 0.05)。依他尼酸和布雷他汀可能是逆转AHSA1基因表达的小分子靶向药物。结论 AHSA1 mRNA可能是HCC潜在的致癌基因, AHSA1 mRNA高水平表达可能促进HCC组织中免疫浸润,并且与HCC患者预后不良相关。
-
关键词:
- 肝细胞癌 /
- 热休克90 kDa蛋白ATPase同源物1的激活因子 /
- 生物信息学 /
- 免疫浸润 /
- 预后
Abstract:Objective To investigate the expression of activator of heat shock 90 kDa protein ATPase homolog 1 (AHSA1) in hepatocellular carcinoma (HCC) and analyze the relationship between AHSA1 expression level and prognosis.Methods HCCDB, GEPIA and Oncomine databases were used to analyze the expression of AHSA1 mRNA in HCC and normal liver tissues. HCC RNA-seq expression and clinical information were downloaded from the Cancer Genome Atlas (TCGA), and univariate and multivariate Cox proportional hazards regression models and Kaplan-Meier plots were used to evaluate the value of AHSA1 in predicting the prognosis of liver cancer. R software was used to construct the nomogram based on the expression level of AHSA1, and the calibration curve was plotted to evaluate the consistency between the actual survival and the predicted survival. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) gene set enrichment analyses were perform to reveal tumor-associated biological processes related to AHSA1. The TIMER2.0 and GFPIA database were used to evaluate the correlation between AHSAl and tumor immune infiltration in HCC. Small molecule targeted drugs acting on AHSA1 were screened by CMAP.Results The expression level of AHSA1 in HCC tissues was significantly higher than that in normal tissues (P < 0.01). High expression of AHSA1 was associated with the poor prognosis of HCC (P < 0.05), and was an independent factor affecting overall survival (OS) (HR=1.970, P < 0.001). The nomogram showed that AHSA1 gene expression was correlated with the risk of HCC, and the area under the curve (AUC) of predicting OS at 1 year, 3 and 5 years were 0.721, 0.711 and 0.725, respectively. The calibration chart showed that the predicted survival rate curve was in good agreement with the actual survival rate curve. GO and KEGG enrichment analysis showed that AHSAl was able to promote tumor progression by mediating neutrophil activation and participating in biological processes such as glycolysis and gluconeogenesis. The expression level of AHSA1 mRNA was associated with the degrees of immune infiltration by B cells, CD4+ T cells, regulatory T cells, macrophages, neutrophils and dendritic cells (P < 0.05). Etacrynic acid and blebbistatin might be the small molecule targeted drugs that can reverse the expression of AHSA1.Conclusion AHSA1 mRNA may be a potential oncogene in HCC, the high expression of AHSA1 mRNA may promote the immune infiltration of HCC tissues, and is associated with poor prognosis of HCC patients. -
Von-Hippel Lindau(VHL)综合征主要表现为中枢神经系统及视网膜血管母细胞瘤(HB), 亦常累及腹部及盆腔内多脏器,其生物学特点为染色体3p25-26上的VHL基因突变引起pVHL抑癌蛋白功能丧失,最终导致肿瘤发生[1]。VHL综合征在临床中较为罕见,诊断时不易与其他中枢神经系统占位性病变相鉴别,最终确诊需结合病史、临床表现、病理活检及基因检测结果。本研究回顾性分析成都市第二人民医院神经外科收治的1例VHL综合征患者的临床资料并进行文献复习,探讨VHL综合征的临床特点及诊疗手段,旨在为该病的临床诊治提供一定参考。
1. 病例资料
患者男性,38岁,因右肩及颈背部持续性胀痛1年收治入院。患者于1年前无明显诱因出现右肩及颈背疼痛,呈持续性胀痛,伴有右手力量减弱,尺侧麻木不适,右上肢上举及下垂时疼痛可加重,不伴有右肩关节活动受限、颈部疼痛等,于外院就诊,行中医康复理疗后未见疼痛、麻木改善而转诊至本院。入院后查体示: 颈椎生理曲度正常,颈部肌肉稍紧张, C5~C6、C6~C7棘间及右侧椎旁压痛,右上肢尺侧浅感觉较对侧减弱,右上肢肌力4级,右下肢及左侧肢体肌力正常,右上肢腱反射亢进,双侧病理征(-), 右侧椎间孔挤压试验(+), 臂丛牵拉试验(-), 数字评分法(NRS)评分6分。术前检验结果未见异常。胸椎磁共振成像(MRI)检查示: 脊髓胸8节段见囊状长T1、长T2不强化低信号影,考虑脊髓空洞,椎体上缘水平胸髓偏左见明显强化结节灶,约0.9 cm, 考虑占位病变,见图 1A、图 1B。腹部MRI检查示: 胰腺增粗,实质内见多发类圆形长T1、长T2信号影,右肾边缘见直径约0.7 cm的长T2信号影,考虑胰腺多发囊肿、弥漫性囊腺瘤、右肾囊肿,见图 1C、图 1D。鉴别诊断: HB的T1加权像(T1WI)表现为低信号囊状肿块,囊壁上可见较小等信号壁结节,T2加权像(T2WI)囊肿表现为高信号,壁结节为等信号,病灶外可有1根或数根较粗大血管伸入病灶,临床可据此与其他肿瘤相鉴别,但最终鉴别仍要依靠病理检查结果。
完善相关检查后考虑椎管内占位性病变,患者于2020年12月7日接受全身麻醉下髓内占位性病变切除术。术中,以传统后入路途径进入胸椎管暴露脊髓,见肿块位于脊髓内偏左,质韧,切开后为灰黄色团块,不易分离,与周围血管粘连紧密,血供丰富,分块瘤内切除后送病理检查,确保肿瘤被完全切除后充分止血,受压的脊髓及神经松解后回位,硬膜下反复冲洗至清亮,创面无渗血,连续缝合硬膜后以生物胶及生物膜贴附硬膜,保证封闭,见图 1E、图 1F。患者术后临床症状缓解,术区无出血及感染。术后病理检查结果示: 可见短梭形细胞及胞浆空亮细胞,可见异型细胞,倾向于间叶源性肿瘤,个别管腔内见中性粒细胞聚集,结合苏木精-伊红(HE)染色形态和其他免疫指标结果,病理诊断支持肿瘤为毛细血管性HB(WHO神经系统肿瘤分级为Ⅰ级),见图 1G。随访复查MRI示: 胸7~8椎体术后改变,并见条片状长T1、长T2信号,部分节段性分布,增强扫描强化不明显,同平面椎管稍变窄,见图 1H。仔细询问病史和家族史得知,患者父亲死于肾癌,留取该患者外周血进行DNA双向测序,结果显示VHL基因的外显子1的第313位碱基A发生点突变(替换为碱基C),导致编码的第105个氨基酸由丝氨酸变为精氨酸,该检测结果与既往研究结论吻合,患者家属未行基因检测。根据患者临床表现、检验检查结果、病理资料及基因检测结果综合考虑,诊断该患者为Ⅰ型VHL综合征。患者出院后随访,未见复发及特殊不适。
2. 讨论
VHL综合征是一种临床较为罕见的疾病,基本病变属常染色体显性遗传性肿瘤,发生率为1/36 000~1/50 000[1]。近70%VHL综合征患者以中枢神经系统HB为首发表现,病变位于脊髓者仅占7%[2]。脊髓HB最常见于颈段,病变位于胸段者则极罕见,本例患者即以胸髓HB为首发,结合家族病史、影像学检查结果(合并肾脏及胰腺囊肿),依据诊断标准明确诊断为Ⅰ型VHL综合征,并建议患者出院后半年复查腹部CT及MRI评估肾脏及胰腺囊肿情况,明确有无手术指征,若需手术治疗则在切除后依据病理结果为该病的诊断提供更加有力的证据[3-4]。
目前, VHL综合征的治疗方法仍以对症处理为主,临床对于中枢神经系统病变尚未有统一的治疗标准,一般仅在出现压迫症状或占位效应时才采取外科手段干预,其中显微外科手术仍是首选治疗方法,而HB对传统的放疗、化疗并不敏感[5]。VHL综合征累及全身多处病灶且易转移,患者常预后不佳,症状明显或瘤体较大者可行手术切除,病灶不大但手术窗口易暴露的肿瘤同样适宜行手术切除,散发型HB患者预后良好,经手术完全切除病灶后可完全治愈。
较大的实性肿瘤由于病灶血供丰富,发展迅速,应在出现症状后尽早手术,但完整切除的难度较大,结合本例患者具体情况而言,可分块切除肿瘤,术中应注意严格止血,或可在术前行血管栓塞术以降低术中出血风险。术中保持术区视野清晰,避免损伤重要血管及脊髓神经是保障手术成功的重要基石,也是积极预防术后出血、功能缺损等并发症发生的必要手段。完全切除肿瘤后应再次检查,对于凝血功能异常而压迫症状较重的限期手术患者,可放置引流管引流,避免术后积血。肿瘤直径在1 cm以下或尚无明显临床表现者,无需特殊处理,随访即可[6-7]。脊髓HB患者术前神经功能缺损在手术切除病灶后多可恢复,其好转程度取决于脊髓受压情况,大多数患者预后尚可。瘤体较小、散在分布多个病灶或难以完全清除的实质肿瘤可采用立体定向放射治疗,有小样本研究[8]证实,立体定向放射治疗对于该类型脊髓HB的效果较好。此外,位置在边缘或体积较小的视网膜HB可采用激光点击和冰冻疗法,病变位于视盘或体积较大的肿瘤则采用手术切除更为安全有效[9]。腹部脏器肿瘤需根据具体情况进行专科评估后再制订治疗方案,无变化或未引起症状的囊肿不必特殊处理,可定期随访观察,肾癌(往往恶性程度较高)、嗜铬细胞瘤(有高血压危象的风险)应早期手术切除[10], 一般可获得良好的预后。
-
表 1-1 正常肝组织和HCC中的不同免疫细胞的免疫标记集与AHSA1的关系
免疫细胞 基因标志物 正常组织 肝癌组织 cor P cor P CD8+ T细胞 CD8A 0.34 0.015 0.25 9.9e-07 CD8B 0.37 0.007 7 0.30 4.0e-09 T细胞 CD3D 0.26 0.063 0.25 9.6e-07 CD3E 0.25 0.075 0.21 4.7e-05 CD2 0.17 0.24 0.22 1.3e-05 B细胞 CD19 0.32 0.025 0.1 0.085 CD79A 0.36 0.01 0.061 0.24 单核细胞 CD86 0.3 0.036 0.39 1.2e-14 CD115 (CSF1R) 0.36 0.009 4 0.34 1.6e-11 肿瘤相关巨噬细胞 CCL2 0.23 0.11 0.021 0.69 CD68 0.42 0.002 5 0.29 8.3e-09 IL10 0.13 0.38 0.41 2.2e-16 M1巨噬细胞 INOS (NOS2) 0.19 0.19 -0.03 0.57 IRF5 0.23 0.11 0.22 2.1e-05 COX2(PTGS2) 0.18 0.2 0.039 0.45 M2巨噬细胞 CD163 0.3 0.037 0.38 3.1e-14 VSIG4 0.32 0.022 0.36 9.6e-13 MS4A4A 0.32 0.024 0.36 7.5e-13 中性粒细胞 CD66b(CEACAM8) 0.25 0.08 0.000 46 0.99 CD11b (ITGAM) 0.43 0.001 7 0.2 8.9e-05 CCR7 0.35 0.014 0.068 0.19 自然杀伤细胞 KIR2DL1 0.32 0.026 0.092 0.079 KIR2DL3 0.21 0.14 0.12 0.019 KIR2DL4 0.25 0.082 0.16 0.001 6 KIR3DL1 0.27 0.061 0.12 0.019 KIR3DL2 0.068 0.64 0.14 0.008 9 KIR3DL3 0.15 0.29 0.1 0.046 KIR2DS4 0.008 9 0.86 0.14 0.33 表 1-2 正常肝组织和HCC中的不同免疫细胞的免疫标记集与AHSA1的关系
免疫细胞 基因标志物 正常组织cor 肝癌组织 cor P cor P 树突状细胞 HLA-DPB1 0.3 0.033 0.24 2.4e-06 HLA-DQB1 0.4 0.004 4 0.22 1.4e-05 HLA-DRA 0.25 0.08 0.25 9.5e-07 HLA-DPA1 0.23 0.11 0.18 0.000 57 BDCA-1(CD1C) 0.28 0.05 0.084 0.11 BDCA-4(NRP1) 0.46 0.000 89 0.36 2e-12 CD11c(ITGAX) 0.16 0.28 0.19 0.000 17 Th1 T-bet (TBX21) 0.4 0.003 6 0.16 0.002 1 STAT4 0.2 0.17 0.014 0.79 STAT1 0.58 1e-05 0.14 0.007 4 IFN-γ(IFNG) 0.27 0.058 0.21 5.9e-05 TNF-α(TNF) 0.21 0.14 0.005 5 0.92 Th2 GATA3 0.13 0.36 0.39 9.3e-15 STAT6 0.69 2.9e-08 0.097 0.063 STAT5A 0.53 6.9e-05 0.19 3e-04 IL13 -0.023 0.88 -0.028 0.59 Tfh BCL6 0.012 0.94 0.078 0.14 IL21 0.19 0.18 0.023 0.66 Th17 STAT3 0.18 0.2 0.099 0.058 IL17A 0.25 0.079 0.029 0.58 调节性T细胞 FOXP3 0.31 0.027 -0.023 0.66 CCR8 0.2 0.16 0.16 0.002 8 STAT5B 0.44 0.001 3 0.1 0.05 TGFβ (TGFB1) 0.38 0.005 8 0.31 6.2e-10 T细胞耗竭 PD-1 (PDCD1) 0.4 0.004 0.14 0.007 CTLA4 0.23 0.11 0.22 2e-05 LAG3 0.21 0.15 0.26 3.6e-07 TIM-3 (HAVCR2) 0.25 0.079 0.19 0.000 18 GZMB 0.36 0.011 5.5e-05 0.21 表 2 CMAP筛选出的可以逆转肝癌细胞AHSA1表达的13种最重要的潜在小分子药物
排序 CMAP名称 mean n 富集分数 P 1 Ly-294002 -0.409 61 -0.445 0.000 01 2 斑鸠霉素(ikarugamycin) 0.606 3 0.930 0.000 62 3 格隆溴铵(glycopyrronium bromide) -0.657 5 -0.799 0.000 70 4 依他尼酸(etacrynic acid) -0.691 3 -0.907 0.001 52 5 硫丙咪胺马来酸(thioperamide) 0.590 5 0.724 0.003 72 6 羟甲唑啉(oxymetazoline) -0.589 4 -0.780 0.004 83 7 布雷他汀(blebbistatin) -0.749 2 -0.952 0.004 89 8 莫米松(mometasone) -0.469 4 -0.773 0.005 39 9 环己米特(cicloheximide) -0.451 4 -0.772 0.005 47 10 吐根碱(emetine) -0.481 4 -0.765 0.006 21 11 辛可宁(cinchonine) -0.242 4 -0.760 0.006 76 12 伏立诺他(vorinostat) -0.324 12 -0.455 0.008 26 13 哌西他嗪(piperacetazine) -0.443 4 -0.741 0.008 81 -
[1] JEMAL A, WARD E M, JOHNSON C J, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival[J]. J Natl Cancer Inst, 2017, 109(9): djx030. http://chdi.wiscweb.wisc.edu/wp-content/uploads/sites/620/2018/06/djx030.pdf
[2] OROZ J, BLAIR L J, ZWECKSTETTER M. Dynamic Aha1 co-chaperone binding to human Hsp90[J]. Protein Sci, 2019, 28(9): 1545-1551. doi: 10.1002/pro.3678
[3] WORTMANN P, GÖTZ M, HUGEL T. Cooperative Nucleotide Binding in Hsp90 and Its Regulation by Aha1[J]. Biophys J, 2017, 113(8): 1711-1718. doi: 10.1016/j.bpj.2017.08.032
[4] HOLMES J L, SHARP S Y, HOBBS S, et al. Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin[J]. Cancer Res, 2008, 68(4): 1188-1197. doi: 10.1158/0008-5472.CAN-07-3268
[5] WOLMARANS A, LEE B, SPYRACOPOULOS L, et al. The Mechanism of Hsp90 ATPase Stimulation by Aha1[J]. Sci Rep, 2016, 6: 33179. doi: 10.1038/srep33179
[6] WANDINGER S K, RICHTER K, BUCHNER J. The Hsp90 chaperone machinery[J]. J Biol Chem, 2008, 283(27): 18473-18477. doi: 10.1074/jbc.R800007200
[7] LIU H J, JIANG X X, GUO Y Z, et al. The flavonoid TL-2-8 induces cell death and immature mitophagy in breast cancer cells via abrogating the function of the AHA1/Hsp90 complex[J]. Acta Pharmacol Sin, 2017, 38(10): 1381-1393. doi: 10.1038/aps.2017.9
[8] ZHENG D, LIU W, XIE W, et al. AHA1 upregulates IDH1 and metabolic activity to promote growth and metastasis and predicts prognosis in osteosarcoma[J]. Signal Transduct Target Ther, 2021, 6(1): 25. doi: 10.1038/s41392-020-00387-1
[9] ZHANG Y, ZHANG Y, LIN Q H. Progesterone-modulated proteins in human endometrial cancer cell line Ishikawa[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2006, 26(8): 1110-1113. http://www.ncbi.nlm.nih.gov/pubmed/16939895
[10] HEIDER M, EICHNER R, STROH J, et al. The IMiD target CRBN determines HSP90 activity toward transmembrane proteins essential in multiple myeloma[J]. Mol Cell, 2021, 81(6): 1170-1186, e10. doi: 10.1016/j.molcel.2020.12.046
[11] KOCHHAR A, KOPELOVICH L, SUE E, et al. p53 modulates Hsp90 ATPase activity and regulates aryl hydrocarbon receptor signaling[J]. Cancer Prev Res (Phila), 2014, 7(6): 596-606. doi: 10.1158/1940-6207.CAPR-14-0051
[12] BAKER-WILLIAMS A J, HASHMI F, BUDZYNSKI M A, et al. Co-chaperones TIMP2 and AHA1 Competitively Regulate Extracellular HSP90: Client MMP2 Activity and Matrix Proteolysis[J]. Cell Rep, 2019, 28(7): 1894-1906, e6. doi: 10.1016/j.celrep.2019.07.045
[13] CAO R, SHAO J, HU Y, et al. microRNA-338-3p inhibits proliferation, migration, invasion, and EMT in osteosarcoma cells by targeting activator of 90 kDa heat shock protein ATPase homolog 1[J]. Cancer Cell Int, 2018, 18: 49. doi: 10.1186/s12935-018-0551-x
[14] KLAUSCHEN F, MVLLER K R, BINDER A, et al. Scoring of tumor-infiltrating lymphocytes: From visual estimation to machine learning[J]. Semin Cancer Biol, 2018, 52(Pt 2): 151-157. http://www.onacademic.com/detail/journal_1000040435720910_e056.html
[15] LIPSON E J, DRAKE C G. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma[J]. Clin Cancer Res, 2011, 17(22): 6958-6962. doi: 10.1158/1078-0432.CCR-11-1595
[16] HELLMANN M D, NATHANSON T, RIZVI H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer[J]. Cancer Cell, 2018, 33(5): 843-852, e4. doi: 10.1016/j.ccell.2018.03.018
[17] ZHANG H H, MEI M H, FEI R, et al. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses[J]. J Viral Hepat, 2010, 17(Suppl 1): 34-43. http://labs.europepmc.org/abstract/MED/20586932
[18] HONG G Q, CAI D, GONG J P, et al. Innate immune cells and their interaction with T cells in hepatocellular carcinoma[J]. Oncol Lett, 2021, 21(1): 57. http://www.researchgate.net/publication/347062334_Innate_immune_cells_and_their_interaction_with_T_cells_in_hepatocellular_carcinoma_Review
[19] LIU J, FAN L, YU H, et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages[J]. Hepatology, 2019, 70(1): 241-258.
[20] FU J, XU D, LIU Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients[J]. Gastroenterology, 2007, 132(7): 2328-2339. doi: 10.1053/j.gastro.2007.03.102
[21] ARIHARA F, MIZUKOSHI E, KITAHARA M, et al. Increase in CD14+ HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis[J]. Cancer Immunol Immunother, 2013, 62(8): 1421-1430. doi: 10.1007/s00262-013-1447-1
[22] LANGHANS B, NISCHALKE H D, KRÄMER B, et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma[J]. Cancer Immunol Immunother, 2019, 68(12): 2055-2066. doi: 10.1007/s00262-019-02427-4
[23] HINDLEY J P, FERREIRA C, JONES E, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors[J]. Cancer Res, 2011, 71(3): 736-746. doi: 10.1158/0008-5472.CAN-10-1797
[24] LU C, RONG D, ZHANG B, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities[J]. Mol Cancer, 2019, 18(1): 130. doi: 10.1186/s12943-019-1047-6
[25] KITAMURA T, QIAN B Z, POLLARD J W. Immune cell promotion of metastasis[J]. Nat Rev Immunol, 2015, 15(2): 73-86. doi: 10.1038/nri3789
[26] BORST J, AHRENDS T, BABAŁA N, et al. CD4(+) T cell help in cancer immunology and immunotherapy[J]. Nat Rev Immunol, 2018, 18(10): 635-647. doi: 10.1038/s41577-018-0044-0
[27] FARHOOD B, NAJAFI M, MORTEZAEE K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review[J]. J Cell Physiol, 2019, 234(6): 8509-8521. doi: 10.1002/jcp.27782
[28] GHOSH S, SHINOGLE H E, GARG G, et al. Hsp90 C-terminal inhibitors exhibit antimigratory activity by disrupting the Hsp90α/Aha1 complex in PC3-MM2 cells[J]. ACS Chem Biol, 2015, 10(2): 577-590. doi: 10.1021/cb5008713
[29] STIEGLER S C, RVBBELKE M, KOROTKOV V S, et al. A chemical compound inhibiting the Aha1-Hsp90 chaperone complex[J]. J Biol Chem, 2017, 292(41): 17073-17083. doi: 10.1074/jbc.M117.797829
[30] PARODY J P, ALVAREZ MDE L, QUIROGA A, et al. Hepatocytes isolated from preneoplastic rat livers are resistant to ethacrynic acid cytotoxicity[J]. Arch Toxicol, 2007, 81(8): 565-573. doi: 10.1007/s00204-007-0183-8
[31] MISHRA M, JAYAL P, KARANDE A A, et al. Identification of a co-target for enhancing efficacy of sorafenib in HCC through a quantitative modeling approach[J]. Febs j, 2018, 285(21): 3977-3992. doi: 10.1111/febs.14641
[32] AL-DALI A M, WEIHER H, SCHMIDT-WOLF I G H. Utilizing ethacrynic acid and ciclopirox olamine in liver cancer[J]. Oncol Lett, 2018, 16(5): 6854-6860. doi: 10.3892/ol.2018.9472/download
[33] ROMAN B I, VERHASSELT S, STEVENS C V. Medicinal Chemistry and Use of Myosin Ⅱ Inhibitor (S)-Blebbistatin and Its Derivatives[J]. J Med Chem, 2018, 61(21): 9410-9428. doi: 10.1021/acs.jmedchem.8b00503
[34] DUXBURY M S, ASHLEY S W, WHANG E E. Inhibition of pancreatic adenocarcinoma cellular invasiveness by blebbistatin: a novel myosin Ⅱ inhibitor[J]. Biochem Biophys Res Commun, 2004, 313(4): 992-997. doi: 10.1016/j.bbrc.2003.12.031
[35] DERYCKE L, STOVE C, VERCOUTTER-EDOUART A S, et al. The role of non-muscle myosin ⅡA in aggregation and invasion of human MCF-7 breast cancer cells[J]. Int J Dev Biol, 2011, 55(7-9): 835-840. http://www.researchgate.net/profile/Christophe_Stove/publication/51871398_The_role_of_non-muscle_myosin_IIA_in_aggregation_and_invasion_of_human_MCF-7_breast_cancer_cells/links/58fe78e945851565029dffc9/The-role-of-non-muscle-myosin-IIA-in-aggregation-and-invasion-of-human-MCF-7-breast-cancer-cells.pdf





 下载:
下载:
 苏公网安备 32100302010246号
苏公网安备 32100302010246号